What Is SOGLIA™?
In Italian, soglia means threshold, or the place of entering or beginning. We think of it as an open door that welcomes those that are “clinical trial curious” and supports them on a path to becoming “clinical trial confident”
SOGLIA Is Different by Design
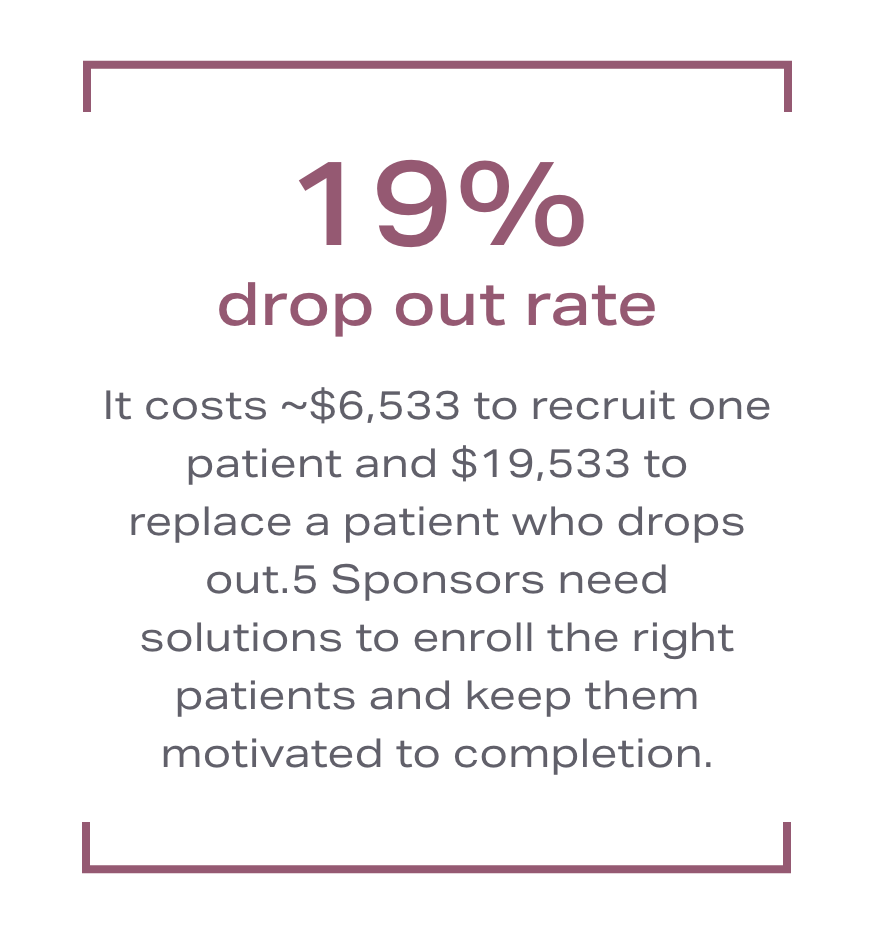
Finding a clinical trial is challenging. A complicated design, coupled with unfamiliar terms creates confusion and leaves patients feeling helpless. SOGLIA is different
As a PXO, we focus on the emotionally intelligent moments that matter. Grounded in human-centered design, we balance the physical and emotional needs of patients with your business requirements.
Through SOGLIA, patients are empowered with the necessary information to make decisions with confidence.
By connecting those who are considering a trial with those who have walked the path before them, we quite literally help them cross the clinical trial threshold.